Lamas, et al. (See below for other contributors)
Background: Chelation therapy, in combination with high dose oral multivitamins and multiminerals (MV), has been used to treat atherosclerotic disease. This analysis of the NIH-funded Trial to Assess Chelation Therapy (TACT) focuses on the comparison of 2 treatment groups chelation + MV versus placebo infusions + placebo MV, evaluating a treatment strategy reflecting clinical practice, and not previously presented in detail.
Methods: TACT, a multi-center, double-blind, placebo-controlled, 2 X 2 factorial trial of EDTA chelation and MV enrolled 1708 patients age  50 years, MI
50 years, MI  6 weeks prior, creatinine
6 weeks prior, creatinine  2.0, assigned to 40 IV chelation or placebo infusions and a 28-component oral MV or placebo. Primary endpoint was death, MI, stroke, coronary revascularization, or hospitalization for angina. Secondary endpoint was cardiovascular mortality, MI, or stroke. We describe the intent-to-treat comparison of 2 factorial groups: chelation + MV (n=421) vs placebo chelation + placebo MV (n=437). Treatment comparisons were by log rank test.
2.0, assigned to 40 IV chelation or placebo infusions and a 28-component oral MV or placebo. Primary endpoint was death, MI, stroke, coronary revascularization, or hospitalization for angina. Secondary endpoint was cardiovascular mortality, MI, or stroke. We describe the intent-to-treat comparison of 2 factorial groups: chelation + MV (n=421) vs placebo chelation + placebo MV (n=437). Treatment comparisons were by log rank test.
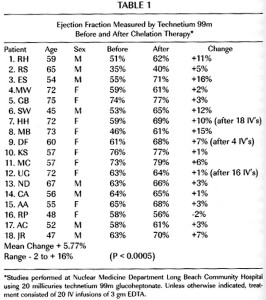
Results: The median age was 65 years, 83% had prior coronary revascularization, and 73% were on statins. Key baseline characteristics were similar between groups. Primary endpoint occurred in 108 (26%) patients in the chelation + MV, and 139 (32%) in the placebo + placebo group (p=0.016). The 5-year Kaplan Meier estimates for the primary endpoint in the chelation + high-dose vitamin group was 31.9%, and in the placebo infusions + placebo vitamin group 40.2%. The secondary endpoint occurred in 39 (9%) of chelation + MV and in 58 (13%) of placebo + placebo (p=0.045). The point estimates for each component of the combined endpoints were all <1.0 and consistent with the overall effect (Table).
Conclusions: In stable patients with a history of MI on appropriate evidence based medical therapy, the beneficial effect of the combination of high-dose vitamins and chelation therapy was statistically significant and of potential clinical relevance.

http://circ.ahajournals.org/cgi/content/meeting_abstract/128/22_MeetingAbstracts/A11054
Gervasio A Lamas1; Richard L Nahin2; Lauren Lindblad3; Christine Goertz4; Robin Boineau5; Terry Chappell6; Greg Flaker7; Theodore Rozema8; Tammy Born9; Mario Stylianou10; Eldrin F Lewis11; Daniel B Mark12; Kerry L Lee3
1 Div of Cardiology, Mount Sinai Med Cntr, Miami Beach, FL
2 31 Cntr Dr, MS 2182, National Cntr for Complementary and Alternative Medicine, Bethesda, MD
3 Biostatistics & Bioinformatics, Duke Clinical Rsch Institute, Durham, NC
4 Chiropractic Rsch, Palmer College of Chiropractic, Davenport, IA
5 HEART FAILURE & ARRHYTHMIAS BRANCH, National Heart, Lung and Blood Institute, Bethesda, MD
6 N/A, Celebration of Heath Association, Bluffton, OH
7 Cardiovascular Medicine, Univ of Missouri Health Care, Columbia, MO
8 N/A, Biogenesis Med Cntr, Landrum, SC
9 N/A, Born Preventive Health Care Clinic, Grand Rapids, MI
10 Biostatistics, National Heart, Lung and Blood Institute, Bethesda, MD
11 Cardiovascular Div, Brigham & Women’s Hosp, Boston, MA
12 Medicine – Cardiology, Duke Clinical Rsch Institute, Durham, NC

 50 years, MI
50 years, MI  2.0, assigned to 40 IV chelation or placebo infusions and a 28-component oral MV or placebo. Primary endpoint was death, MI, stroke, coronary revascularization, or hospitalization for angina. Secondary endpoint was cardiovascular mortality, MI, or stroke. We describe the intent-to-treat comparison of 2 factorial groups: chelation + MV (n=421) vs placebo chelation + placebo MV (n=437). Treatment comparisons were by log rank test.
2.0, assigned to 40 IV chelation or placebo infusions and a 28-component oral MV or placebo. Primary endpoint was death, MI, stroke, coronary revascularization, or hospitalization for angina. Secondary endpoint was cardiovascular mortality, MI, or stroke. We describe the intent-to-treat comparison of 2 factorial groups: chelation + MV (n=421) vs placebo chelation + placebo MV (n=437). Treatment comparisons were by log rank test.